Changes in steel when heated
Most of the heat treatment of steel needs to be heated to obtain austenite, and then cooled at different speeds to transform the austenite into different structures to obtain different properties of the steel. Therefore, to master the law of heat treatment, we must first study the changes of steel during heating.
The formation process of austenite when heated
1. Heating transformation of eutectoid steel
It can be seen from the iron-carbon phase diagram that when the steel is heated to a temperature above 727°C (the PSK line of the state diagram, also known as the A1 temperature), pearlite transforms into austenite. This heating rate is very slow, and the actual heating rate of heat treatment is higher than this slow heating rate. The actual transformation temperature of pearlite to austenite is higher than A1, and the actual transformation temperature is defined as Ac1. Ac1 is higher than A1, indicating that there is thermal hysteresis, the faster the heating rate, the higher the Ac1, and the shorter the time to complete the transformation from pearlite to austenite.
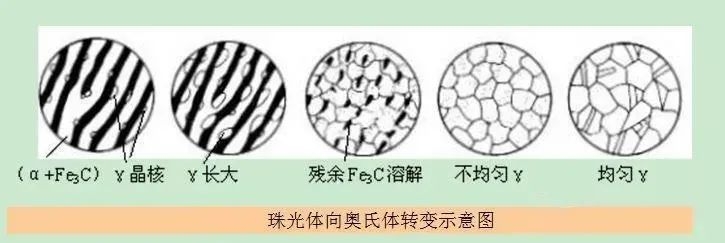
Eutectoid carbon steel (containing 0.77%C) has pearlite structure before heating, generally a lamellar structure with ferrite phase and cementite phase arranged alternately. The austenite transformation process during heating can be divided into four steps. ,As shown below.
2. Heating transformation of non-eutectoid steels
The first stage: the formation of austenite nuclei. It is known from the Fe-Fe3C state diagram: at the A1 temperature, the ferrite contains about 0.0218%C, the cementite contains 6.69%C, and the austenite contains 0.77%C. During the transformation of pearlite into austenite, the original ferrite is reorganized from the body-centered cubic lattice to the face-centered cubic lattice of austenite, and the original cementite is transformed from the complex orthorhombic lattice to the face-centered cubic lattice. . Therefore, the heating transformation of steel has both the diffusion of carbon atoms and the change of crystal structure. Based on energy and composition conditions, austenite nuclei are generated at the interface between the ferrite and cementite phases of pearlite (see Figure (a)).
The second stage: the growth of austenite. After the austenite nucleus is formed, one side of it is connected to cementite and the other side is connected to ferrite. With the transformation of ferrite (the reduction of the ferrite area) and the dissolution of the cementite (the reduction of the cementite area), the austenite continues to expand to the original ferrite area and the cementite area on both sides. Grow up until the ferrite completely disappears and the austenite meets each other to form austenite grains one by one.
Three stages: dissolution of residual cementite. Since the rate of ferrite transformation into austenite is much higher than the dissolution rate of cementite, there are still a lot of undissolved "residual cementite" after the complete transformation of ferrite (see Figure (C)), and also It takes a certain period of time to keep the cementite to dissolve.
Four stages: homogenization of the austenite composition. Even if the cementite is completely dissolved, the composition of the austenite is still uneven. It needs to be kept for a sufficient time to allow the carbon atoms to fully diffuse, and the austenite composition may be uniform.
The above analysis shows that there must be two necessary and sufficient conditions for the transformation of pearlite into austenite and the uniform composition of austenite: one is the temperature condition, which must be heated above Ac1, and the other is the time condition, which requires sufficient temperature above Ac1. time. Under the condition of a certain heating rate, the higher the temperature exceeding Ac1, the shorter the time required for the formation of austenite and the homogenization of the composition; under the condition of a certain temperature (higher than Ac1), the longer the holding time, the longer the austenite composition more evenly.
It should also be seen that the change of austenite grains from small size to large size is a spontaneous process. At a certain heating temperature above Ac1, too long a holding time will lead to the merger of austenite grains and a larger size. In contrast, for the same heating time, the increasing tendency of austenite grain size caused by high heating temperature is significantly greater than that of austenite grain growth at low heating temperature. The austenite grain size is too large (or too coarse), which often leads to the reduction of the strength of the steel after heat treatment. In engineering, it is often desirable to obtain fine and uniform austenite grains. The lowest possible austenitization temperature is selected under the condition of uniform composition; the second way is to rapidly heat to a higher temperature, and then short-term heat preservation makes the formed austenite too late to grow and cool to obtain fine grains.
In engineering, the size of austenite grains is defined as grain size, and is divided into 8 grades, of which grades 1 to 4 are coarse grains, grades 5 and above are fine grains, and grades over 8 are ultra-fine grains.
The transformation process of pearlite into austenite in hypoeutectoid steel and hypereutectoid steel is the same as the transformation process of eutectoid steel, that is, the pearlite in hypoeutectoid steel or hypereutectoid steel is heated above the Ac1 temperature. to be transformed into austenite. The difference is the transformation of ferrite of hypoeutectoid steel and the dissolution of secondary cementite of hypereutectoid steel. More importantly, the complete transformation of ferrite should be above the A3 temperature (GS line of the Fe-Fe3C state diagram), considering that the thermal hysteresis should actually be above Ac3, and the complete dissolution of the secondary cementite should be at the temperature Acm (Fe- The ES line of the Fe3C state diagram) above, considering the thermal hysteresis should be above Accm. That is to say, the microstructure of hypoeutectoid steel after heating should be all austenite above Ac3, and for hypereutectoid steel, it should be above Accm. If the hypoeutectoid steel is still heated only between the temperature of Ac1 and Ac3, no matter how long the heating time is, the microstructure after heating is still coexistence of ferrite and austenite. For hypereutectoid steel heated between Ac1 ~ Accm temperature, the structure after heating should be the coexistence of secondary cementite and austenite. The microstructure transformation in the cooling process after heating is only the transformation from austenite to other microstructures, and the ferrite and secondary cementite will not be transformed during the cooling process.

Common defects when steel is heated
(1) Oxidation
The oxidizing atmosphere (such as air, O2, CO2, H2O, etc.) during heating oxidizes the steel and forms FeO, Fe2O3, Fe3O4 and other oxides on the surface of the workpiece. At temperatures below 560 °C, relatively dense oxides such as Fe3O4 are mainly formed, which can isolate the steel surface from the oxidizing atmosphere and prevent further oxidation of the steel surface. However, the austenitization temperature of steel is mostly above 560 °C, and the steel is oxidized to form a loose oxide layer mainly composed of FeO. The thickness of the oxide layer increases with the increase of heating temperature and heating time, which not only leads to the burning loss of steel Increase the size, and make the size of the part smaller, the surface is rough, and more importantly, it seriously affects the quality of the subsequent heat treatment.
(2) Decarbonization
Decarburization during the heating process of steel, that is, the phenomenon that the carbon in the steel is burned and the carbon content on the steel surface is reduced. Decarburization often occurs along with oxidation, and the oxidizing atmosphere is also a decarburizing atmosphere. Although H2 is a reducing atmosphere, it is also a decarburizing atmosphere. Generally, the higher the carbon content in steel, the more serious the decarburization is. Due to decarburization, the carbon content on the surface of the steel decreases, resulting in a decrease in the mechanical strength of the steel, especially the fatigue strength of the workpiece and the wear resistance.
(3) Overheating
The overheating of steel means that the heating temperature is higher than the normal temperature, and the phenomenon is that the austenite grains of the steel are larger than normal, that is, the grains become coarser. As a result, the plasticity, toughness and strength of the steel are reduced, and the deformation of the workpiece after heat treatment increases, which may also lead to heat treatment cracks and scrap the workpiece. The overheated workpiece can generally be reheated at a lower temperature to re-fine the austenite grains and be remedied.
(4) Overburning
It refers to the phenomenon that the heating temperature is too high, and the austenite grain boundary or part of the grain boundary is oxidized or even melted. The consequence is that the workpiece to be processed is very brittle. If it is forged, it will crack, and the burnt workpiece can only be scrapped and cannot be saved, so it is fatal.
How to prevent heating defects:
(1) Vacuum heating
Heating the workpiece in vacuum is an effective measure to prevent oxidative decarburization, and it is the development direction of heat treatment technology. It is widely used in developed countries. The problem is that the equipment investment for vacuum heating is large and the process cost is high.
(2) Controlled atmosphere heating
During the heating process of the workpiece, a certain protective atmosphere is filled into the furnace to ensure that the steel is heated in an atmosphere without decarburization, carbon increase and oxidation. Practice has proved that it is an effective and reliable method, and it is also a very common process in developed countries, and it is one of the development directions of modern heat treatment. However, a set of generating devices for producing a controllable atmosphere is required, and its application is limited due to the high cost and limited source of raw materials.
(3) Salt bath heating
The workpiece is heated in a melted neutral salt solution, and the salt solution is fully deoxidized to ensure less or even no oxidation of the workpiece during the heating process. The main problem is that the salt that sticks to the workpiece is difficult to clean, and if the cleaning is not clean, it will lead to rust during storage and application. In addition, during the operation, the salt solution is easy to explode in contact with water, and it is easy to burn the human body if you are not careful, so you must pay great attention to safety.


